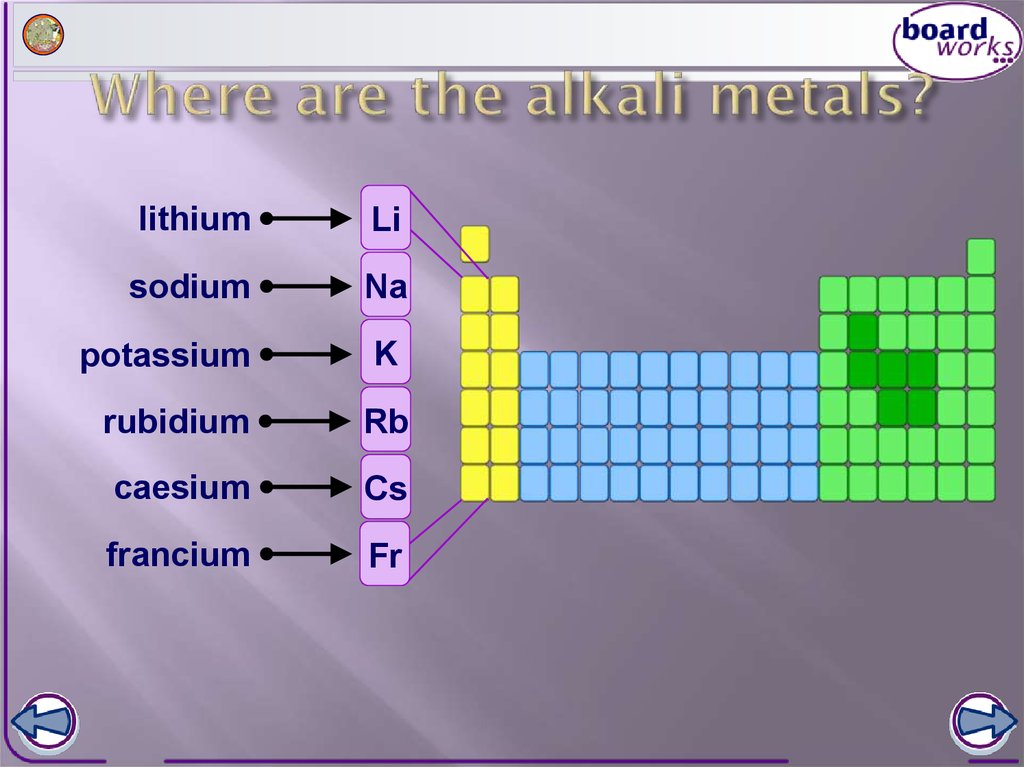
Properties And Uses Of Alkali Metals . In keeping with overall periodic trends, the. The alkali metals are on. The alkali metals tend to form +1 cations. The key characteristic these elements share in common is that they all have one. The atomic properties of alkali metals reflect the relatively high energy and large size of their ns valence orbitals. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The alkali metals are the elements located in group ia of the periodic table (the first column). The alkali metals have the high thermal and electrical conductivity, lustre, ductility, and. Elements in the first column of the periodic table. Various properties of the group 1 elements are summarized in table \(\pageindex{1}\). General properties of the alkali metals.
from ppt-online.org
The alkali metals tend to form +1 cations. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. In keeping with overall periodic trends, the. Various properties of the group 1 elements are summarized in table \(\pageindex{1}\). The atomic properties of alkali metals reflect the relatively high energy and large size of their ns valence orbitals. The alkali metals are on. Elements in the first column of the periodic table. The alkali metals are the elements located in group ia of the periodic table (the first column). General properties of the alkali metals. The alkali metals have the high thermal and electrical conductivity, lustre, ductility, and.
The alkali metals презентация онлайн
Properties And Uses Of Alkali Metals Various properties of the group 1 elements are summarized in table \(\pageindex{1}\). The alkali metals are the elements located in group ia of the periodic table (the first column). The alkali metals are on. The key characteristic these elements share in common is that they all have one. General properties of the alkali metals. Various properties of the group 1 elements are summarized in table \(\pageindex{1}\). The atomic properties of alkali metals reflect the relatively high energy and large size of their ns valence orbitals. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. Elements in the first column of the periodic table. The alkali metals have the high thermal and electrical conductivity, lustre, ductility, and. In keeping with overall periodic trends, the. The alkali metals tend to form +1 cations.
From online-learning-college.com
Group 1 alkali metals Properties of alkali metals Reactions Properties And Uses Of Alkali Metals The key characteristic these elements share in common is that they all have one. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The alkali metals have the high thermal and electrical conductivity, lustre, ductility, and. The alkali metals are on. The alkali metals. Properties And Uses Of Alkali Metals.
From www.tes.com
Lesson Alkali Metals GCSE Edexcel 91 Teaching Resources Properties And Uses Of Alkali Metals The alkali metals tend to form +1 cations. Elements in the first column of the periodic table. The alkali metals are the elements located in group ia of the periodic table (the first column). The alkali metals are on. General properties of the alkali metals. In keeping with overall periodic trends, the. The atomic properties of alkali metals reflect the. Properties And Uses Of Alkali Metals.
From www.vrogue.co
The General Properties Of The Alkali Metals In The Mo vrogue.co Properties And Uses Of Alkali Metals The key characteristic these elements share in common is that they all have one. In keeping with overall periodic trends, the. Elements in the first column of the periodic table. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The alkali metals tend to. Properties And Uses Of Alkali Metals.
From overallscience.com
Trends in atomic and physical properties of alkali metals Overall Science Properties And Uses Of Alkali Metals General properties of the alkali metals. The alkali metals tend to form +1 cations. The atomic properties of alkali metals reflect the relatively high energy and large size of their ns valence orbitals. The alkali metals are on. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation). Properties And Uses Of Alkali Metals.
From byjus.com
Alkali Metals Properties, Electronic Configuration, Periodic Trends Properties And Uses Of Alkali Metals In keeping with overall periodic trends, the. Elements in the first column of the periodic table. The key characteristic these elements share in common is that they all have one. The alkali metals have the high thermal and electrical conductivity, lustre, ductility, and. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it. Properties And Uses Of Alkali Metals.
From studylib.net
Alkali metals Properties And Uses Of Alkali Metals In keeping with overall periodic trends, the. The alkali metals are the elements located in group ia of the periodic table (the first column). The alkali metals tend to form +1 cations. Elements in the first column of the periodic table. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to. Properties And Uses Of Alkali Metals.
From ppt-online.org
The alkali metals презентация онлайн Properties And Uses Of Alkali Metals The alkali metals are the elements located in group ia of the periodic table (the first column). Elements in the first column of the periodic table. General properties of the alkali metals. The alkali metals are on. The atomic properties of alkali metals reflect the relatively high energy and large size of their ns valence orbitals. In keeping with overall. Properties And Uses Of Alkali Metals.
From www.nagwa.com
Question Video Identifying the Property of Alkali Metals From a List Properties And Uses Of Alkali Metals Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. In keeping with overall periodic trends, the. The alkali metals have the high thermal and electrical conductivity, lustre, ductility, and. The alkali metals tend to form +1 cations. The key characteristic these elements share in. Properties And Uses Of Alkali Metals.
From newtondesk.com
Alkaline Earth Metals On The Periodic Table Chemistry Elements Properties And Uses Of Alkali Metals The alkali metals are the elements located in group ia of the periodic table (the first column). The alkali metals are on. The atomic properties of alkali metals reflect the relatively high energy and large size of their ns valence orbitals. General properties of the alkali metals. Cation formation is favored by the relatively low ionization energies of the free. Properties And Uses Of Alkali Metals.
From scienceinfo.com
Comparison of properties of Alkali and Alkaline Earth Metals Properties And Uses Of Alkali Metals General properties of the alkali metals. The atomic properties of alkali metals reflect the relatively high energy and large size of their ns valence orbitals. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The key characteristic these elements share in common is that. Properties And Uses Of Alkali Metals.
From studylib.net
'alkali metals'? Properties And Uses Of Alkali Metals The alkali metals have the high thermal and electrical conductivity, lustre, ductility, and. The atomic properties of alkali metals reflect the relatively high energy and large size of their ns valence orbitals. Various properties of the group 1 elements are summarized in table \(\pageindex{1}\). General properties of the alkali metals. Elements in the first column of the periodic table. In. Properties And Uses Of Alkali Metals.
From ppt-online.org
The alkali metals презентация онлайн Properties And Uses Of Alkali Metals In keeping with overall periodic trends, the. Various properties of the group 1 elements are summarized in table \(\pageindex{1}\). The alkali metals are on. The key characteristic these elements share in common is that they all have one. General properties of the alkali metals. The alkali metals have the high thermal and electrical conductivity, lustre, ductility, and. The alkali metals. Properties And Uses Of Alkali Metals.
From www.vedantu.com
Alkali Metals Chemical Elements, Properties Alkali Metals Periodic Properties And Uses Of Alkali Metals Elements in the first column of the periodic table. The alkali metals tend to form +1 cations. The alkali metals are the elements located in group ia of the periodic table (the first column). The atomic properties of alkali metals reflect the relatively high energy and large size of their ns valence orbitals. In keeping with overall periodic trends, the.. Properties And Uses Of Alkali Metals.
From elchoroukhost.net
Alkali Metals Periodic Table Reactivity Elcho Table Properties And Uses Of Alkali Metals General properties of the alkali metals. The alkali metals are on. Elements in the first column of the periodic table. The key characteristic these elements share in common is that they all have one. The atomic properties of alkali metals reflect the relatively high energy and large size of their ns valence orbitals. In keeping with overall periodic trends, the.. Properties And Uses Of Alkali Metals.
From elchoroukhost.net
Alkali Metals Periodic Table Location Elcho Table Properties And Uses Of Alkali Metals Various properties of the group 1 elements are summarized in table \(\pageindex{1}\). Elements in the first column of the periodic table. The alkali metals tend to form +1 cations. General properties of the alkali metals. The atomic properties of alkali metals reflect the relatively high energy and large size of their ns valence orbitals. The alkali metals are on. The. Properties And Uses Of Alkali Metals.
From www.online-sciences.com
Alkali metals compounds properties and uses (Sodium hydroxide and Properties And Uses Of Alkali Metals General properties of the alkali metals. The atomic properties of alkali metals reflect the relatively high energy and large size of their ns valence orbitals. The alkali metals are the elements located in group ia of the periodic table (the first column). The alkali metals have the high thermal and electrical conductivity, lustre, ductility, and. In keeping with overall periodic. Properties And Uses Of Alkali Metals.
From www.youtube.com
Properties of the Alkaline Earth Metals YouTube Properties And Uses Of Alkali Metals The alkali metals have the high thermal and electrical conductivity, lustre, ductility, and. In keeping with overall periodic trends, the. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. General properties of the alkali metals. The alkali metals are on. The alkali metals are. Properties And Uses Of Alkali Metals.
From knordslearning.com
Alkaline Earth Metals Periodic Table (With Images) Properties And Uses Of Alkali Metals General properties of the alkali metals. The alkali metals are on. The atomic properties of alkali metals reflect the relatively high energy and large size of their ns valence orbitals. The alkali metals are the elements located in group ia of the periodic table (the first column). The alkali metals have the high thermal and electrical conductivity, lustre, ductility, and.. Properties And Uses Of Alkali Metals.